Introduction
Synaptic transmission and sensory processing in the inner ear and auditory pathway
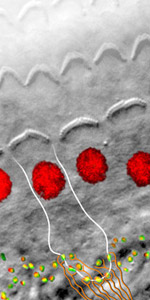
The InnerEarLab studies the physiology, pathophysiology and restoration of sensory processing at the synapses of the mammalian inner ear and the auditory pathway. Synaptic transmission at the hair cell ribbon synapses with the spiral ganglion neurons of the cochlea and their endbulb of Held with bushy cells of the cochlear nucleus operates with submillisecond temporal precision and drives neural spiking at hundreds per second over hours. These auditory synapses are morphologically specialized: the hair cell synapse features a single and large ribbon-type active zone and the endbulb of Held is a large, craw-like or calyceal presynaptic terminal with more than one hundred small “conventional” active zones. When hair cells transduce a sound driven mechanical stimulus into an electrical signal, voltage-gated Ca2+ channels open and the Ca2+ influx triggers exocytosis of glutamate filled vesicles at the ribbon synapses. Our hypothesis is that the hair cell ribbon synapse bears a highly advanced molecular machinery that is highly specialized and deviates from the make-up of small CNS synapses. Each of the 5-20 active zones drives spiking in one spiral ganglion neuron in the absence and presence of acoustic stimulation. High and sustained rates of synaptic transmission require very efficient means of vesicle cycling. It becomes evident that interfering with the hair cell vesicle cycle results in auditory dysfunction, be it vesicle docking, priming, fusion or endocytic membrane retrieval. Moreover, the synapses of one hair cell differ from each other in structure and function, most probably in order to cover the broad range of sound pressures with the limited dynamic range of encoding at each individual synapse. The endbulb of Held Synapse acts as a reliable and temporally precise relay synapse. 3-5 endbulbs likely arising from different spiral ganglion neurons collectively drive the bushy cell, which acts as a coincidence detector..
InnerEarLab

The InnerEarLab brings together a variety of approaches to perform a detailed analysis of the anatomy and physiology of hair cell ribbon synapses and endbulb of Held synapses from the molecular level up to systems physiology and behavior. The "Molecular Architecture of Synapses" group of Carolin Wichmann combines immunohistochemistry, high resolution light microscopy, and electron microscopy to study the molecular architecture of synapses and how structure relates to function. Using electron microscopic techniques as electron tomography and high-pressure freezing/freeze-substitution (HPF/FS) the group studies morphological aspects of wild-type and mutant synapses qualitatively and quantitatively. The combination of defined stimulation followed by rapid HPF/FS and finally electron tomography allows one to determine synaptic vesicle pools, docking or tethering of synaptic vesicles in high spatio-temporal resolution under different conditions (resting or stimulated). The "Molecular anatomy, physiology and pathology of sound encoding" group of Tobias Moser employs pre- and postsynaptic patch-clamp, membrane capacitance measurements, imaging of Ca2+ and vesicle cycling, photolysis of caged compounds and biophysical modeling to study ribbon synapse function at the level of single hair cells or single hair cell synapse. More recently, the group also started studying the endbulb of Held synapse. The group collaborates with the groups of Stefan Hell and Carolin Wichmann to study the molecular nanoanatomy of these synapses using confocal, STED, 4Pim and electron microscopy. The goal is to relate structure and function of auditory synapses in normal and mutant mice. The group of Christian Vogl studies the development of inner hair cell ribbon synapses using advanced light microscopy and molecular biology techniques. The group of Tina Pangršič Vilfan studies the molecular and cellular physiology of vestibular neurotransmission and noise-induced hearing loss. The group uses pre- and postsynaptic patch-clamp, membrane capacitance measurements and photolysis of caged compounds to study the synapses of hair cells in normal and mutant mice. The group of Nicola Strenzke studies auditory systems physiology at the single neuron and population levels recording otoacoustic emissions, cochlear potentials as well as auditory nerve and brainstem neuronal responses to sound. Startle responses and behavioral studies are pursued to flank the systems physiology approach to hair cell synaptic dysfunction.